Abstract
BACKGROUND
Pediatric immune thrombocytopenia (ITP) is an acquired disorder of platelet destruction that is associated with an increased risk of bleeding. Despite published guidelines for the management of ITP, the available evidence is of low grade, leading to practice variation in different settings. The use of validated bleeding scores to guide clinical decision making is inconsistent. In addition, many children are initially treated with medications despite the recommendation for observation in newly diagnosed children with ITP and no or mild bleeding symptoms. This approach leads to over-utilization of healthcare resources including hospitalizations, medication administration, and medical encounters for management-related side effects. In 2020, a quality improvement (QI) project of the Pediatric ITP Consortium of North America (ICON) was initiated to improve consistency in clinical practice at ICON sites using national ITP guidelines.
DESIGN/METHODS
Within the ICON QI subcommittee, a standardized clinical care pathway (Figure 1) for newly diagnosed childhood ITP was developed based on the American Society of Hematology (ASH) 2019 guidelines. The goal was to unify approach to management, decrease practice variation, identify and learn from deviations in decision making, and decrease resource utilization by increasing observation rates in low-risk pediatric ITP patients. Site investigators shared the care pathway to update institutional providers on national guidelines. For Aim 1 of this project, sites completed a multi-center, retrospective analysis documenting the pre-QI pathway management of children, ages 1-16 years, diagnosed with ITP from January to December 2019. Statistical analysis was performed using R version 4.0.2. For Aim 2, after local dissemination and education of the clinical care pathway, clinicians at all participating sites will review the pathway at the time of managing newly diagnosed children and then complete a short survey documenting a bleeding score and management decisions.
RESULTS
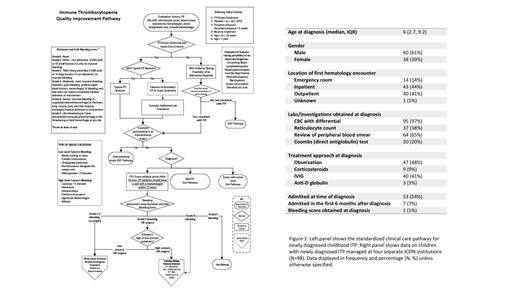
Current data from the retrospective review is summarized in Figure 1. 98 patients across four ICON institutions are included in this analysis. The median age at diagnosis was 6 years (IQR 2.7, 9.2) with 61% being male. 43 (44%) patients had their first hematology encounter in the inpatient setting, 40 (41%) in the outpatient clinic, and 14 (14%) in the emergency room. Buchanan and Adix bleeding scores were obtained from only one patient (1%) at diagnosis. Treatment strategies varied including observation in 47 (48%) patients, IVIG in 40 (41%), corticosteroids in 9 (9%), and anti-D globulin in 3 (3%). 53 (54%) patients were admitted at the time of diagnosis. The prospective QI pathway is being utilized by six ICON institutions and 20 patients have been followed on the pathway since November 2020. An additional seven sites are in various phases of study activation.
DISCUSSION
Evidence-based ITP guidelines and an expert consensus report have been recently published. For children with newly diagnosed ITP and a platelet count <20 x 10 9/L who have no or mild bleeding, ASH guidelines suggest against admission to the hospital and suggest observation rather than treatment with corticosteroids. Retrospective analysis of the management at four ICON centers demonstrates the variation in approach to treatment. However, although guidelines suggest initial management based on objective assessment of bleeding symptoms, only one patient (1%) had a documented bleeding score at presentation, suggesting a lack of a standard approach to management and practice variation. These data support the need for this quality initiative, which involves clinicians reviewing the pathway while managing patients and answering a survey at the time of clinical visits to report on bleeding symptoms and management. This initiative will be expanded to include a total of 13 institutions across the United States. Data will be analyzed every 1-2 years and changes will be made to the pathway with the goal of improving care. Further quality initiatives may help to standardize the management approach of pediatric ITP patients and optimize health outcomes in this patient population.
Badawy: Bluebird Bio Inc: Consultancy; Sanofi Genzyme: Consultancy; Vertex Pharmaceuticals Inc: Consultancy. Grace: Novartis: Research Funding; Dova: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Research Funding; Principia: Membership on an entity's Board of Directors or advisory committees. Nakano: Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal